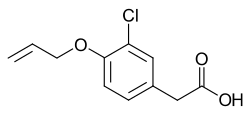
Alclofenac
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.709 |
| Chemical and physical data | |
| Formula | C11H11ClO3 |
| Molar mass | 226.66 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Alclofenac is a nonsteroidal anti-inflammatory drug (NSAID).
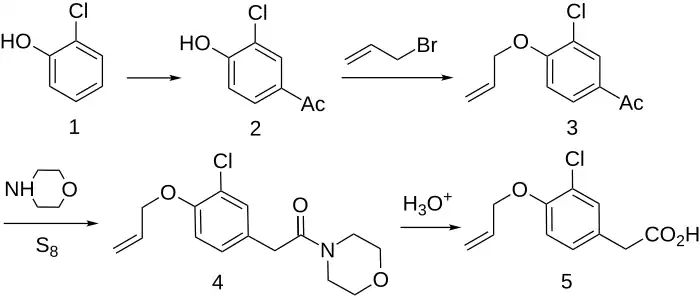
Synthesis
References
- ^ Gaviraghi G, Banfi S, Cornelli U, Pinza M, Pifferi G (April 1977). "Synthesis and antiinflammatory activity of 3-chloro-4-cyclopropylmethoxyphenylacetic acid and its alpha-methyl homologue". Il Farmaco; Edizione Scientifica. 32 (4): 286–95. PMID 862882.
- ^ CA 831040, Gillet C, Buu HN, Lambelin G, "Substituted Phenylacetic Acids and Processes of Preparation Thereof", published 30 December 1969, assigned to Madan Ag
- ^ US 3824277, Gillet C, Buu HN, Lambelin G, "4-Allyloxy-3-chloro-phenyl-acetic acid", published 16 July 1974, assigned to Madan Ag
| pyrazolones / pyrazolidines | |
|---|---|
| salicylates | |
| acetic acid derivatives and related substances | |
| oxicams |
|
| propionic acid derivatives (profens) |
|
| n-arylanthranilic acids (fenamates) | |
| COX-2 inhibitors (coxibs) | |
| other | |
| NSAID combinations | |
Key: underline indicates initially developed first-in-class compound of specific group; #WHO-Essential Medicines; †withdrawn drugs; ‡veterinary use. | |
| Receptor (ligands) |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enzyme (inhibitors) | |||||||||||||||||
| Others | |||||||||||||||||
| |||||||||||||||||
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.