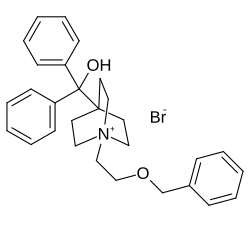
Umeclidinium bromide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Incruse Ellipta |
| Other names | GSK573719A |
| License data | |
| Routes of administration | Inhalation (DPI) |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | ~89%[3] |
| Metabolism | Liver (CYP2D6) |
| Elimination half-life | 11 hours |
| Excretion | Feces (58%) and urine (22%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.166.375 |
| Chemical and physical data | |
| Formula | C29H34BrNO2 |
| Molar mass | 508.500 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Umeclidinium bromide, sold under the brand name Incruse Ellipta among others, is a long-acting muscarinic antagonist approved for the maintenance treatment of chronic obstructive pulmonary disease (COPD).[3] It is also approved for this indication in combination with vilanterol (as umeclidinium bromide/vilanterol),[5][6][7] and also as a triple-therapy combination as fluticasone furoate/umeclidinium bromide/vilanterol.[8]
It is on the World Health Organization's List of Essential Medicines.[9] In 2020, it was the 245th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[10][11]
References
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2014". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 10 April 2023.
- ^ "Product monograph brand safety updates". Health Canada. 7 July 2016. Retrieved 3 April 2024.
- ^ a b c "Incruse Ellipta- umeclidinium aerosol, powder". DailyMed. 13 December 2023. Retrieved 28 March 2025.
- ^ "Rolufta Ellipta (previously Rolufta)". European Medicines Agency (EMA). 20 March 2017. Retrieved 28 March 2025.
- ^ "Anoro Ellipta- umeclidinium bromide and vilanterol trifenatate powder". DailyMed. 2 June 2023. Retrieved 28 March 2025.
- ^ Feldman GJ, Edin A (December 2013). "The combination of umeclidinium bromide and vilanterol in the management of chronic obstructive pulmonary disease: current evidence and future prospects". Therapeutic Advances in Respiratory Disease. 7 (6): 311–9. doi:10.1177/1753465813499789. PMID 24004659. S2CID 5744282.
- ^ "FDA Approves Umeclidinium and Vilanterol Combo for COPD". Medscape. December 18, 2013.
- ^ "Trelegy Ellipta- fluticasone furoate, umeclidinium bromide and vilanterol trifenatate powder". DailyMed. 2 June 2023. Retrieved 28 March 2025.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ^ "Umeclidinium - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.