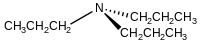
Tripropylamine
| Names | |
|---|---|
| IUPAC name
N,N-Dipropylpropan-1-amine
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.771 |
| EC Number |
|
| 101540 | |
PubChem CID
|
|
| UNII | |
| UN number | 2260 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Appearance | colorless liquid |
| Density | 0.7558 g/cm3 |
| Melting point | −93.5 °C (−136.3 °F; 179.7 K) |
| Boiling point | 156 °C (313 °F; 429 K) |
| Hazards | |
| GHS labelling:[1] | |
| |
| Danger | |
| H226, H301, H311, H314, H332, H335 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P262, P264, P264+P265, P270, P271, P273, P280, P301+P316, P301+P330+P331, P302+P352, P302+P361+P354, P303+P361+P353, P304+P340, P305+P354+P338, P316, P317, P319, P321, P330, P361+P364, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Tripropylamine is an organic compound with the formula (CH3CH2CH2)3N. It is classified as a tertiary amine. It is a colorless liquid with a "fishy" odor.[2]
It has been used in electrochemiluminescence as a coreactant.[3]

References
- ^ "Tripropylamine". pubchem.ncbi.nlm.nih.gov.
- ^ Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2000). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a02_001. ISBN 9783527303854.
- ^ Hanif, Saima; Han, Shuang; John, Peter; Gao, Wenyue; Kitte, Shimeles Addisu; Xu, Guobao (April 2016). "Electrochemiluminescence of Luminol-Tripropylamine System". Electrochimica Acta. 196: 245–251. doi:10.1016/j.electacta.2016.02.175.