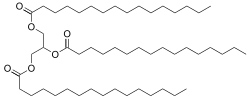
Tripalmitin
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Propane-1,2,3-triyl tri(hexadecanoate) | |
| Other names
Palmitin; Glycerol tripalmitate; Glycerin tripalmitate; Glyceryl tripalmitate; Palmitic triglyceride; Tripalmitoyl glycerol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.272 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C51H98O6 | |
| Molar mass | 807.339 g·mol−1 |
| Appearance | White powder |
| Density | 0.8752 g/cm3 (70 °C)[1] |
| Melting point | 44.7–67.4 °C (112.5–153.3 °F; 317.8–340.5 K)[2][3] |
| Boiling point | 315 °C (599 °F; 588 K) at 760 mmHg[1] |
| Insoluble | |
| Solubility | Soluble in EtOH, (C2H5)2O, C6H6, CHCl3[1] |
Refractive index (nD)
|
1.4381 (80 °C)[1] |
| Structure | |
| Triclinic (β-form)[4] | |
| P1 (β-form)[4] | |
| Thermochemistry | |
Heat capacity (C)
|
1219.4 J/mol·K (β-form, 281.2 K) 1753.1 J/mol·K (338.8 K)[3][5] |
Std molar
entropy (S⦵298) |
1387.4 J/mol·K (liquid)[5] |
Std enthalpy of
formation (ΔfH⦵298) |
−2468.7 kJ/mol[5] |
Std enthalpy of
combustion (ΔcH⦵298) |
−31605.9 kJ/mol[5] |
| Hazards | |
| GHS labelling: | |
| |
| Warning | |
| H411 | |
| P273, P391, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Tripalmitin is a triglyceride derived from the fatty acid palmitic acid.
References
- ^ a b c d Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ Hong, Jindui (2010). "Solid−Liquid−Gas Equilibrium of the Ternaries Ibuprofen + Myristic Acid + CO2and Ibuprofen + Tripalmitin + CO2". Journal of Chemical & Engineering Data. 55 (1): 297–302. doi:10.1021/je900342a.
- ^ a b Charbonnet, G. H.; Singleton, W. S. (1947). "Thermal properties of fats and oils". Journal of the American Oil Chemists' Society. 24 (5): 140. doi:10.1007/BF02643296. S2CID 101805872.
- ^ a b Van Langevelde, A.; Van Malssen, K.; Hollander, F.; Peschar, R.; Schenk, H. (1999). "Structure of mono-acid even-numbered β-triacylglycerols". Acta Crystallographica Section B. 55 (Pt 1): 114–122. Bibcode:1999AcCrB..55..114V. doi:10.1107/S0108768198009392. PMID 10927345.
- ^ a b c d Tripalmitin in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD) (retrieved 2014-06-19)
- ^ "MSDS of Trimyristin". fishersci.ca. Fisher Scientific. Retrieved 2014-06-19.
