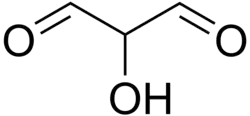
Glucic acid
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Hydroxypropanedial | |
| Other names
Hydroxymalonaldehyde
2-Hydroxypropanedial Reductone Tartronaldehyde 2-Hydroxymalonaldehyde 2-Hydroxymalondialdehyde Glucose-reductone Tartronal Tartronic aldehyde Triose reductone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H4O3 | |
| Molar mass | 88.062 g·mol−1 |
| Density | 1.38 g/mL |
| Melting point | 149 °C (300 °F; 422 K) (decomposes)[1] |
| Boiling point | 274 °C (525 °F; 547 K) |
| Related compounds | |
Related alkenals
|
4-Hydroxynonenal |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Glucic acid is an acid produced by the action of acids on cane-sugar or of alkalis on glucose.
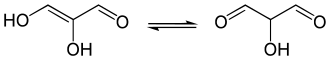
Tautomeric forms of glucic acid
References
- ^ Holker, J. R. (1955). "Oxidation of Some Enediols with Selenium Dioxide". J. Chem. Soc.: 579–580. doi:10.1039/JR9550000574.