Sodium ethanethiolate
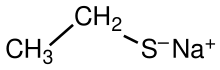 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H5NaS | |
| Molar mass | 84.11 g·mol−1 |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Sodium ethanethiolate is an organosulfur compound with the formula CH3CH2SNa. It is the sodium salt of the conjugate base of ethanethiol. This compound is commercially available as a white solid that is soluble in polar organic solvents. Sodium ethanethiolate is a reagent in organic synthesis. Hydrolysis of sodium ethanethiolate, e.g. in humid air, produces ethanethiol, which has a low odor threshold and a noxious "rotten egg" smell.
Preparation
Sodium ethanethiolate can be produced by treating a solution of ethanethiol with sodium hydride:[1]
- CH3CH2SH + NaH → CH3CH2SNa + H2
The closely related sodium methanethiolate can be prepared and used in situ (i.e., without isolation) by treatment of a solution of methanethiol with strong base such as sodium hydroxide.[2][3]
Reactions
Sodium ethanethiolate is a source of ethanethiolate, a powerful nucleophile. It is used to cleave methoxy-aryl ethers:[1]
- NaSCH2CH3 + Ar−O−CH3 → Ar−ONa + CH3CH2SCH3 (Ar = aryl)
It converts alkyl halides to ethyl thioethers
Oxidation of sodium methanethiolate gives diethyldisulfide:
- 2 NaSCH2CH3 + I2 → CH3CH2SSCH2CH3 + 2 NaI
References
- ^ a b Mirrington, R. N.; Feutrill, G. I. (1873). "Orcinol Monomethyl Ether". Organic Syntheses. 533: 90. doi:10.15227/orgsyn.053.0090.
- ^ Bruce W. Erickson (1974). "γ-Hydroxy-α,β-unsaturated Aldehydes via 1,3-Bis(methylthio)allyllithium: trans-4-Hydroxy-2-hexenal". Organic Syntheses. 54: 19. doi:10.15227/orgsyn.054.0019.
- ^ Cogolli, P.; Maiolo, F.; Testaferri, L.; Tingoli, M.; Tiecco, M. (1979). "Nucleophilic Aromatic substitution reactions of unactivated aryl halides with thiolate ions in hexamethylphosphoramide". J. Org. Chem. 44 (15): 2462. doi:10.1021/jo01329a011.