Perinone
 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.022.363 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
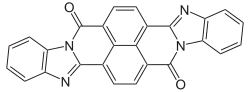
| C26H12N4O2 | |
| Molar mass | 412.408 g·mol−1 |
| Appearance | Orange solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Perinone is a class of organic compounds. The parent compound has two isomers, each of which are useful pigments.
It is prepared from naphthalenetetracarboxylic dianhydride by condensation with o-phenylenediamine. The two Isomers of perinone[1] are useful pigments. The trans isomer is called Pigment Orange 43 ("PO43", CID 78141 from PubChem) and the cis isomer is called Pigment Red 194 ("PR194", CID 77892 from PubChem).[2] Like some structurally related compounds perinone is also an organic semiconductor.[3]
References
- ^ Mizuguchi, Jin (2004). "Crystal Structure and Electronic Characterization of trans-and cis-Perinone Pigments". J. Phys. Chem. B. 108 (26): 8926–8930. doi:10.1021/jp031351d.
- ^ K. Hunger. W. Herbst "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a20_371
- ^ Feast, W. James; Peace, Richard J.; Sage, Ian C.; Wood, Emma L. (March 1999). "Poly(4-vinyltriphenylamine): synthesis and application as a hole transport layer in light-emitting diodes". Polymer Bulletin. 42 (2): 167–174. doi:10.1007/s002890050449. S2CID 95994481.