Perfluoroethylamine
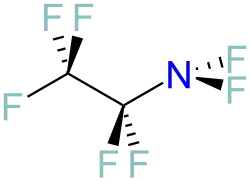 | |
| Names | |
|---|---|
| Preferred IUPAC name
Heptafluoroethanamine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2F7N | |
| Molar mass | 171.02 g/mol |
| Appearance | colorless gas |
| Melting point | −38.1 °C (−36.6 °F; 235.1 K) |
| Boiling point | −183 °C (−297.4 °F; 90.1 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Perfluoroethylamine is an organofluoride. It is perfluorinated ethylamine. Like other N-F containing compounds, it is obscure. Small amounts are formed by the reaction of tetrafluoroethylene and nitrogen trifluoride.[1]
References
- ^ Takagi, Toshiyuki; Tamura, Masanori; Shibakami, Motonari; Quan, Heng-Dao; Sekiya, Akira (2000). "The synthesis of perfluoroamine using nitrogen trifluoride". Journal of Fluorine Chemistry. 101 (1): 15–17. Bibcode:2000JFluC.101...15T. doi:10.1016/S0022-1139(99)00191-8.