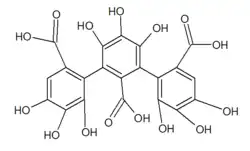
Nonahydroxytriphenic acid
Names
Preferred IUPAC name
14 ,15 ,16 ,23 ,24 ,25 ,34 ,35 ,36 -Nonahydroxy[11 ,21 :23 ,31 -terphenyl]-12 ,22 ,32 -tricarboxylic acid
Other names
Nonahydroxytriphenoyl
Identifiers
ChemSpider
InChI=1S/C21H14O15/c22-5-1-3(19(31)32)7(14(26)12(5)24)9-11(21(35)36)10(17(29)18(30)16(9)28)8-4(20(33)34)2-6(23)13(25)15(8)27/h1-2,22-30H,(H,31,32)(H,33,34)(H,35,36)
Key: KLKCDGZVVKFBQP-UHFFFAOYSA-N
Oc2c(c1c(cc(O)c(O)c1O)C(=O)O)c(c(c(O)c2O)c3c(cc(O)c(O)c3O)C(=O)O)C(=O)O
Properties
C 21 H 14 O 15
Molar mass
−1
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Nonahydroxytriphenic acid is a moiety found in some ellagitannins such as roburin A , B,C and D, castalagin or grandinin .[ 1]
References
^ Roburin A, a dimeric ellagitannin from heartwood of Quercus robur. Hervé Du Penhoat, Michon V. M. F., Ohassan A., Shuyun Peng, Scalbert A. and Gage D., Phytochemistry, 1991, vol. 30, no 1, pages 329-332, INIST 19775624
Moieties Lactones Monomers
Acetonyl geraniin
Alnusiin Bicornin Carlesiin
Casuarictin Emblicanin A and B
Euscaphinin
Galloyl pedunculagin
Grandinin Helioscopinin B
Jolkinin
Lagerstannin A, B and C
Macranganin
Myrobalanitannin
Nupharin A , B, C, D, E and FPedunculagin Punicalagin Punigluconin Phyllanemblinin A, B, C, D, E and F
Punicalin Roburin E
Rugosin E
Sanguiin H-5
Stenophyllanin A , B and CStrictinin Tellimagrandin I and II Teracatain
Terchebulin
Terflavin A and B
Tergallic acid Tergallic acid dilactone C-glycosidic ellagitannins Dehydroellagitannins (molecules withdehydrohexahydroxydiphenic acid (DHHDP) Transformed ellagitannins
molecules with chebulic acid molecules with Elaeocarpusinic acid
Elaeocarpusin
Helioscopin B
Mallojaponin (1-O-Galloyl-2,4-elaeocarpusinoyl-3,6-(R)-valoneayl-beta-D-glucose)
Oligomers Other