Iridium tetrafluoride
 | |
| Names | |
|---|---|
| Other names
Iridium tetrafluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| IrF4 | |
| Molar mass | 268.2109 g/mol |
| Appearance | dark brown solid |
| Related compounds | |
Other anions
|
iridium dioxide |
Other cations
|
sodium fluoride, potassium fluoride, caesium fluoride, calcium fluoride |
Related compounds
|
iridium(V) fluoride iridium(VI) fluoride rhodium(IV) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
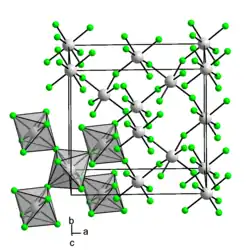
Iridium(IV) fluoride is a chemical compound of iridium and fluorine, with the chemical formula IrF4 and is a dark brown solid.[1] Early reports of IrF4 prior to 1965 are questionable and appear to describe the compound IrF5.[1] The solid can be prepared by reduction of IrF5 with iridium black[1] or reduction with H2 in aqueous HF.[2] The crystal structure of the solid is notable as it was the first example of a three-dimensional lattice structure found for a metal tetrafluoride and subsequently RhF4, PdF4 and PtF4 have been found to have the same structure.[3] The structure has 6 coordinate, octahedral, iridium where two edges of the octahedra are shared and the two unshared fluorine atoms are cis to one another.[3]
References
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. doi:10.1016/C2009-0-30414-6. ISBN 978-0-08-037941-8.
- ^ Paine, Robert T.; Asprey, Larned B. (1975). "Reductive syntheses of transition metal fluoride compounds. Synthesis of rhenium, osmium, and iridium pentafluorides and tetrafluorides". Inorg. Chem. 14 (5): 1111–1113. doi:10.1021/ic50147a030.
- ^ a b Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6