Triazinane
 | |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| 8477997 | |
| ChEBI |
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C3H9N3 | |
| Molar mass | 87.126 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
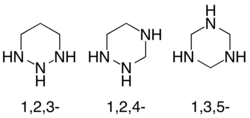
Triazinanes are a class of nitrogen-containing heterocycles.[1] Triazinanes have six-membered cyclohexane-like ring but with three carbon centers replaced by nitrogen. The parent have the molecular formula (CH2)3(NH)3. Three isomeric forms are possible, but only 1,3,5-triazinanes are common. 1,3,5-triazinane is a labile intermediate in the formation of hexamethylenetetramine from ammonia and formaldehyde.
Stable 1,3,5-Triorganotriazines are formed by condensation of primary amines and formaldehyde.[2]
See also
- 6-membered rings with one nitrogen atom: Piperidine
- 6-membered rings with two nitrogen atoms: Diazinane
- Hexahydropyrimidine
- Hexahydropyridazine
- Triazine
- Borazine (borazole)
- Triazenes, organic compound with the formula RN=N−NR2
References
- ^ Heterocyclic Chemistry T.L. Gilchrist 1985 ISBN 0-582-01421-2 (1997, ISBN 0-582-27843-0
- ^ Nielsen, Arnold T.; Atkins, Ronald L.; Moore, Donald W.; Scott, Robert; Mallory, Daniel; LaBerge, Jeanne M. (1973). "Structure and chemistry of the aldehyde ammonias. 1-Amino-1-alkanols, 2,4,6-trialkyl-1,3,5-hexahydrotriazines, and N,N-dialkylidene-1,1-diaminoalkanes". J. Org. Chem. 38 (19): 3288–3295. doi:10.1021/jo00959a010.