Dichloro(1,2-bis(diphenylphosphino)ethane)nickel
 | |
.jpg) | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.157.667 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C26H24Cl2NiP2 | |
| Molar mass | 528.02 g·mol−1 |
| Appearance | orange solid |
| Density | 1.406 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
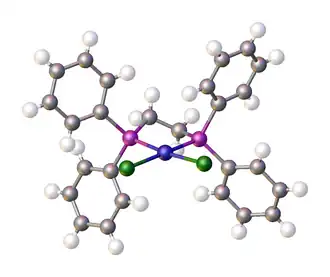
Dichloro[1,2-bis(diphenylphosphino)ethane]nickel is a coordination complex with the formula NiCl2(dppe); where dppe is the diphosphine 1,2-bis(diphenylphosphino)ethane. It is used as a reagent and as a catalyst.[1] The compound is a bright orange-red diamagnetic solid. The complex adopts a square planar geometry.[2][3]
It is prepared by combining equimolar portions of nickel(II) chloride hexahydrate with dppe:
- Ni(H2O)6Cl2 + dppe → NiCl2(dppe) + 6 H2O
See also
References
- ^ Kumada, Makota; Tamao, Kohei; Sumitani, Koji (1978). "Phosphine-Nickel Complex Catalyzed Cross-Coupling of Grignard Reagents with Aryl and Alkenyl Halides: 1,2-Dibutylbenzene". Organic Syntheses. 58: 127. doi:10.15227/orgsyn.058.0127.
- ^ Van Hecke, Gerald R.; Horrocks, Jr., William DeW. (1966). "Ditertiary Phosphine Complexes of Nickel. Spectral, Magnetic, and Proton Resonance Studies. A Planar-Tetrahedral Equilibrium". Inorganic Chemistry. 5 (11): 1968–1974. doi:10.1021/ic50045a029.
- ^ Davison, J. C.; Foreman, M. R. St.-J.; Howie, R. A.; Plater, M.J.; Skakle, J. M. S. (2001). "A New Polymorph, Form C, of [1,2-Bis(diphenylphosphino)ethane]dichloronickel(II)". Acta Crystallogr. C. 57 (6): 690–693. doi:10.1107/S0108270101003961. PMID 11408672.