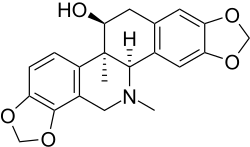
Corynoline
Names
IUPAC name
13-Methylchelidonine
Systematic IUPAC name
(5bR ,6S ,12bR )-5b,13-Dimethyl-5b,6,7,12b,13,14-hexahydro-2H ,10H -[1,3]benzodioxolo[5,6-c ][1,3]dioxolo[4,5-h ]phenanthridin-6-ol
Identifiers
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.208.689
EC Number
UNII
InChI=1S/C21H21NO5/c1-21-14-3-4-15-19(27-10-24-15)13(14)8-22(2)20(21)12-7-17-16(25-9-26-17)5-11(12)6-18(21)23/h3-5,7,18,20,23H,6,8-10H2,1-2H3/t18-,20+,21-/m0/s1
Key: IQUGPRHKZNCHGC-TYPHKJRUSA-N
InChI=1/C21H21NO5/c1-21-14-3-4-15-19(27-10-24-15)13(14)8-22(2)20(21)12-7-17-16(25-9-26-17)5-11(12)6-18(21)23/h3-5,7,18,20,23H,6,8-10H2,1-2H3/t18-,20+,21-/m0/s1
Key: IQUGPRHKZNCHGC-TYPHKJRUBF
O1c2c(OC1)c3c(cc2)[C@@]5([C@H](N(C3)C)c4cc6OCOc6cc4C[C@@H]5O)C
Properties
C 21 H 21 N O 5
Molar mass
−1
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Corynoline is an acetylcholinesterase inhibitor isolated from Corydalis incisa [ 1]
References
Enzyme (modulators)
ChAT Tooltip Choline acetyltransferase
Inhibitors: 1-(-Benzoylethyl)pyridinium2-(α-Naphthoyl)ethyltrimethylammonium
3-Chloro-4-stillbazole
4-(1-Naphthylvinyl)pyridine
Acetylseco hemicholinium-3
Acryloylcholine
AF64A B115
BETA
CM-54,903
N,N-Dimethylaminoethylacrylate
N,N-Dimethylaminoethylchloroacetate AChE Tooltip Acetylcholinesterase BChE Tooltip Butyrylcholinesterase
Transporter (modulators )
CHT Tooltip Choline transporter VAChT Tooltip Vesicular acetylcholine transporter
Release (modulators )
See also
Receptor/signaling modulators
Muscarinic acetylcholine receptor modulators
Nicotinic acetylcholine receptor modulators