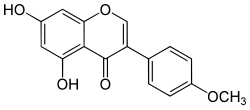
Biochanin A
Names
IUPAC name
5,7-Dihydroxy-4′-methoxyisoflavone
Systematic IUPAC name
5,7-Dihydroxy-3-(4-methoxyphenyl)-4H -1-benzopyran-4-one
Other names
Biochanin
Identifiers
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.007.041
KEGG
UNII
InChI=1S/C16H12O5/c1-20-11-4-2-9(3-5-11)12-8-21-14-7-10(17)6-13(18)15(14)16(12)19/h2-8,17-18H,1H3
Y Key: WUADCCWRTIWANL-UHFFFAOYSA-N
Y InChI=1/C16H12O5/c1-20-11-4-2-9(3-5-11)12-8-21-14-7-10(17)6-13(18)15(14)16(12)19/h2-8,17-18H,1H3
Key: WUADCCWRTIWANL-UHFFFAOYAM
O=C\1c3c(O/C=C/1c2ccc(OC)cc2)cc(O)cc3O
Properties
C 16 H 12 O 5
Molar mass
−1
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Biochanin A is an O -methylated isoflavonephytochemicals known as flavonoids. Biochanin A can be found in red clover [ 1] soy , in alfalfa sprouts, in peanuts , in chickpea (Cicer arietinum ) and in other legumes.
Biochanin A is classified as a phytoestrogen and has putative benefits in dietary cancer prophylaxis . It has also been found to be a weak inhibitor of fatty acid amide hydrolase in vitro [ 2]
The enzyme biochanin-A reductase uses dihydrobiochanin A and NADP+ to produce biochanin A, NADPH, and H+ . The enzyme isoflavone-7-O-beta-glucoside 6"-O-malonyltransferase uses malonyl-CoA and biochanin A 7-O-β-D -glucoside to produce CoA and biochanin A 7-O-(6-O-malonyl-β-D -glucoside).
See also
References
^ Medjakovic, S.; Jungbauer, A. (2008). "Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor". The Journal of Steroid Biochemistry and Molecular Biology . 108 (1– 2): 171– 177. doi :10.1016/j.jsbmb.2007.10.001 . PMID 18060767 . S2CID 206495959 . ^ Thors L, Burston JJ, Alter BJ, McKinney MK, Cravatt BF, Ross RA, Pertwee RG, Gereau RW, Wiley JL, Fowler CJ (2010). "Biochanin A, a naturally occurring inhibitor of fatty acid amide hydrolase" . British Journal of Pharmacology . 160 (3): 549– 560. doi :10.1111/j.1476-5381.2010.00716.x . PMC 2931556 PMID 20590565 .
Receptor (ligands )
CB1 Tooltip Cannabinoid receptor type 1
Agonists(abridged, Inverse agonists Antagonists
CB2 Tooltip Cannabinoid receptor type 2
Agonists
2-AG 2-AGE (noladin ether) 3,3'-Diindolylmethane 4-O-Methylhonokiol α-Amyrin · β-Amyrin A-796,260 A-834,735 A-836,339 AM-1172
AM-1221 AM-1235 AM-1241 AM-2232 Anandamide AZ-11713908 Cannabinol Caryophyllene CB-13 CBS-0550 CP 55,940 GW-405,833 (L-768,242) GW-842,166X HU-308 JTE 7-31 JWH-007 JWH-015 JWH-018 JWH-73
JWH-133 L-759,633 L-759,656 Lenabasum (anabasum) Magnolol MDA-19 Nabitan NADA Olorinab (APD-371) PF-03550096 S-444,823 SER-601 Serinolamide A UR-144 Tedalinab THC (dronabinol) THCV Tetrahydromagnolol
Virodhamine Antagonists
NAGly GPR18 )
GPR55
GPR119
Transporter (modulators )
eCBTs Tooltip Endocannabinoid transporter
Enzyme (modulators)
FAAH Tooltip Fatty acid amide hydrolase MAGL ABHD6
Inhibitors: JZP-169JZP-430
KT182
KT185
KT195
KT203
LEI-106
ML294
ML295
ML296
UCM710
WWL-70 ABHD12
Others
Others: 2-PG (directly potentiates activity of 2-AG at CB1 receptor) ARN-272 (FAAH-like anandamide transporter inhibitor)
See also
Receptor/signaling modulators Cannabinoids (cannabinoids by structure)
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel
3β,5α-Dihydrolevonorgestrel
3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin, pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown
See also
Receptor/signaling modulators
Estrogens and antiestrogens
Androgen receptor modulators
Progesterone receptor modulators
List of estrogens
ERRα Tooltip Estrogen-related receptor alpha ERRβ Tooltip Estrogen-related receptor beta
Agonists: DY-131 (GSK-9089)GSK-4716 (GW-4716)
SLU-PP-332 ERRγ Tooltip Estrogen-related receptor gamma
See also
Receptor/signaling modulators