Acylfulvene
 | |
| Names | |
|---|---|
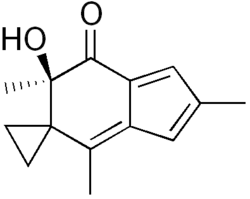
| Preferred IUPAC name
(6′R)-6′-Hydroxy-2′,4′,6′-trimethylspiro[cyclopropane-1,5′-inden]-7′(6′H)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H16O2 | |
| Molar mass | 216.28 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Acylfulvene is a class of cytotoxic semi-synthetic derivatives of illudin, a natural product that can be extracted from the jack o'lantern mushroom (Omphalotus olearius).[1] One important acylfulvene, 6-hydroxymethylacylfulvene (irofulven), has been evaluated for the treatment of a wide assortment of cancers and tumors.[2] It is thought that acylfulvene compounds kill cancer cells by DNA alkylation (see DNA methylation).[3]
References
- ^ Anchel, M.; Hervey, A.; Robbins, W. J. Proc. Natl. Acad. Sci. U.S.A. 1950, 36, 300
- ^ "Search Details". Clinical Trials. Retrieved 22 March 2023.
- ^ Woynarowski, Jan M.; Napier, Cheryl; Koester, Steven K.; Chen, Shih-Fong; Troyer, Dean; Chapman, William; Macdonald, John R. Biochemical Pharmacology (1997), 54 (11), 1181-1193 CODEN: BCPCA6; ISSN 0006-2952. (Elsevier Science Inc.)