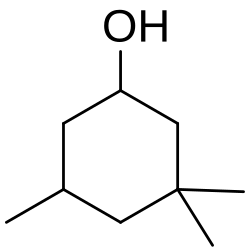
3,3,5-Trimethylcyclohexanol
 | |
| Names | |
|---|---|
| IUPAC name
3,3,5-trimethylcyclohexan-1-ol
| |
| Other names
Homomenthol
| |
| Identifiers | |
3D model (JSmol)
|
|
| 2203314 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.748 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H18O | |
| Molar mass | 142.242 g·mol−1 |
| Density | 0.878 at 20 °C |
| Melting point | 37.0 °C (98.6 °F; 310.1 K) |
| Boiling point | 198 °C (388 °F; 471 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H412 | |
| P264, P273, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
3,3,5-Trimethylcyclohexanol is a precursor to the vasodilator cyclandelate, the sunscreen component homosalate and the VP nerve agent.[1][2] It can be synthesized by hydrogenation of isophorone.[3] It has a mint flavour.
See also
References
- ^ Bell, GD; Clegg, RJ; Ellis, WR; Middleton, B; White, DA (January 1984). "The effects of 3,5,5-trimethylcyclohexanol on hepatic cholesterol synthesis, bile flow and biliary lipid secretion in the rat". British Journal of Pharmacology. 81 (1): 183–7. doi:10.1111/j.1476-5381.1984.tb10759.x. PMC 1986967. PMID 6704580.
- ^ "3-Pyridyl phosphonates". US3903098A.
- ^ "Fragrance raw materials monographs". Food and Cosmetics Toxicology. 12 (7–8): 1007. December 1974. doi:10.1016/0015-6264(74)90227-2.