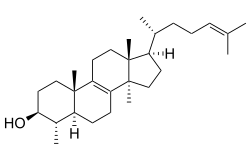
29-Norlanosterol
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(3S,4S,5S,10S,13R,14R,17R)-4,10,13,14-tetramethyl-17-[(2R)-6-methylhept-5-en-2-yl]-1,2,3,4,5,6,7,11,12,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-ol | |
| Other names
4α,14α-Dimethyl-5α-cholesta-8,24-dien-3β-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C29H48O | |
| Molar mass | 412.702 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
29-Norlanosterol, or 4-demethyllanosterol, also called 4α,14α-dimethylzymosterol, is a Metabolic intermediate of plant sterol biosynthesis. In the pathway, it is transformed from ring-opening reactions of norcycloartenol and then demethylation by CYP51 into 4α-methyl-5α-cholesta-8,14,24-trien-3β-ol.[1][2]
References
- ^ Bak, S; Kahn, RA; Olsen, CE; Halkier, BA (February 1997). "Cloning and expression in Escherichia coli of the obtusifoliol 14 alpha-demethylase of Sorghum bicolor (L.) Moench, a cytochrome P450 orthologous to the sterol 14 alpha-demethylases (CYP51) from fungi and mammals". The Plant Journal. 11 (2): 191–201. doi:10.1046/j.1365-313x.1997.11020191.x. PMID 9076987.
- ^ "Rosa chinensis plant sterol biosynthesis II". pmn.plantcyc.org.