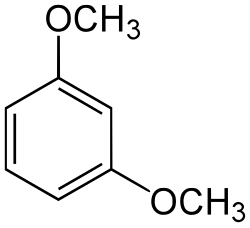
1,3-Dimethoxybenzene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,3-Dimethoxybenzene
| |||
| Other names
Dimethylresorcinol
Resorcinol dimethyl ether | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.259 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H10O2 | |||
| Molar mass | 138.166 g·mol−1 | ||
| Related compounds | |||
Related compounds
|
1,2-Dimethoxybenzene; 1,4-Dimethoxybenzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |||
1,3-Dimethoxybenzene is an organic compound with the formula C6H4(OCH3)2. It is one of three isomers of dimethoxybenzene.
Uses
1,3-Dimethoxybenzene has been used in the synthesis of novel oxathiane spiroketal donors.[1]
Related compounds
References
- ^ A. Fascione, Martin; J. Webb, Nicola; A. Kilner, Colin; L. Warriner, Stuart; Bruce Turnbull, W. (2011-12-28). "Stereoselective glycosylations using oxathiane spiroketal glycosyl donors". Merck. 348: 6–13. doi:10.1016/j.carres.2011.07.020. PMID 22200482.