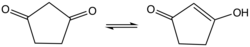1,3-Cyclopentanedione
| Names | |
|---|---|
| Preferred IUPAC name
Cyclopentane-1,3-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| 1362728 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.021.249 |
| EC Number |
|
| 200797 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Appearance | white solid |
| Density | 1.37 g/cm3 |
| Melting point | 149–151 °C (300–304 °F; 422–424 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
1,3-Cyclopentanedione is an organic compound with the formula (CH2)3(CO)2. It is one of two isomeric cyclopentanediones, the other being 1,2-cyclopentanedione. The enol is predicted to be about 1-3 kcal/mol more stable than the diketo form.[1] The enol structure has been confirmed by X-ray crystallography.[2]
Preparation
The compound is prepared by hydrogenation of 2-cyclopentene-1,4-dione using zinc/acetic acid.[3][4]
References
- ^ Jana, Kalyanashis; Ganguly, Bishwajit (2018). "DFT Study to Explore the Importance of Ring Size and Effect of Solvents on the Keto–Enol Tautomerization Process of α- and β-Cyclodiones". ACS Omega. 3 (7): 8429–8439. doi:10.1021/acsomega.8b01008. PMC 6644555. PMID 31458971.
- ^ Katrusiak, A. (1990). "Structure of 1,3-cyclopentanedione". Acta Crystallographica Section C: Crystal Structure Communications. 46 (7): 1289–1293. Bibcode:1990AcCrC..46.1289K. doi:10.1107/S0108270189011352.
- ^ McIntosh, John M.; Beaumier, Pierre. (1972). "Improved Preparation of 1,3-cyclopentanedione". The Journal of Organic Chemistry. 37 (18): 2905–2906. doi:10.1021/jo00983a027.
- ^ Gary H. Rasmusson; Herbert O. House; Edward F. Zaweski; Charles H. DePuy (1962). "2-Cyclopentene-1,4-Dione". Organic Syntheses. 42: 36. doi:10.15227/orgsyn.042.0036.