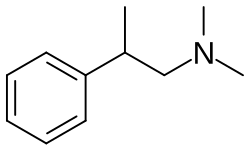
β,N,N-Trimethylphenethylamine
 | |
| Clinical data | |
|---|---|
| Other names | N,N-Dimethyl-2-phenylpropan-1-amine; N,N-DMPPA; DMPPA; β,N,N-TMPEA |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H17N |
| Molar mass | 163.264 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
β,N,N-Trimethylphenethylamine, also known as N,N-dimethyl-2-phenylpropan-1-amine (N,N-DMPPA or DMPPA) is a monoamine releasing agent of the phenethylamine family.[1] It is the N-methyl derivative of phenpromethamine (β,N-methylphenethylamine) and the N,N-dimethyl derivative of β-methylphenethylamine.[1] The drug is a partial norepinephrine releasing agent, with an EC50Tooltip half-maximal effective concentration of 1,337 nM and an EmaxTooltip maximal efficacy of 67% in rat brain synaptosomes.[1] Conversely, it was inactive on dopamine, whereas serotonin was not reported.[1] The drug produces non-significant increases in blood pressure in rodents, but does not affect heart rate or affect locomotor activity.[1] It may be rapidly metabolized and thereby inactivated.[1] DMPPA was first described in the scientific literature by at least 2019.[1]
See also
References
- ^ a b c d e f g Schindler CW, Thorndike EB, Rice KC, Partilla JS, Baumann MH (June 2019). "The Supplement Adulterant β-Methylphenethylamine Increases Blood Pressure by Acting at Peripheral Norepinephrine Transporters". The Journal of Pharmacology and Experimental Therapeutics. 369 (3): 328–336. doi:10.1124/jpet.118.255976. PMC 6533570. PMID 30898867.
External links
| Phenethylamines |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphetamines |
| ||||||||||||||||
| Phentermines |
| ||||||||||||||||
| Cathinones |
| ||||||||||||||||
| Phenylisobutylamines (and further-extended) | |||||||||||||||||
| Catecholamines (and close relatives) |
| ||||||||||||||||
| Cyclized phenethylamines |
| ||||||||||||||||
| Related compounds |
| ||||||||||||||||
| |||||||||||||||||